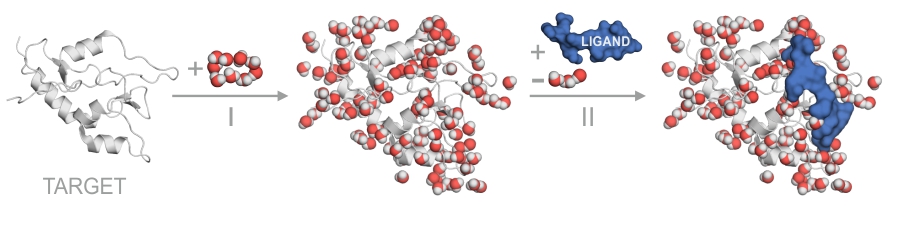
Background
Hydration is involved in most biological processes. It is remarked in a prominent text book on protein structure and function (Petsko and Ringe 2009) that “… waters in fixed positions should be considered as part of the tertiary structure, and any detailed structure description that does not include them is incomplete”. Surface hydration is key determinant of solubility and aggregation of solute molecules (Israelachvili and Wennerström 1996). Protein-ligand interactions are also largely affected by interfacial water molecules (Baron et al. 2012), and therefore, knowledge of their location is of primary importance during structure-based drug design. Whereas resolving hydration structure is important, it is also a very difficult task and there is no ultimate method for determination of hydration structure at atomic level.
The difficulties come from mobility and complexity of interactions of water molecules located on a molecular surface. Residence of a water molecule on the surface is affected not primarily by the strength of its protein-water interaction. It is “rather a topography that prevents the water molecule from exchanging by a cooperative mechanism” (Halle 2004a). Importantly, such a cooperative mechanism of exchange also includes several water-water interactions often detected (Finney 1977) between surface or interface water molecules. Thus, it is very problematic to predict the residence of water molecules in the hydration layer of a protein using merely thermodynamic or kinetic approaches (Halle 2004a).
A brief outlook is provided in the forthcoming sections on available experimental and theoretical methods for determination of hydration structure placing an emphasis on their limitations.